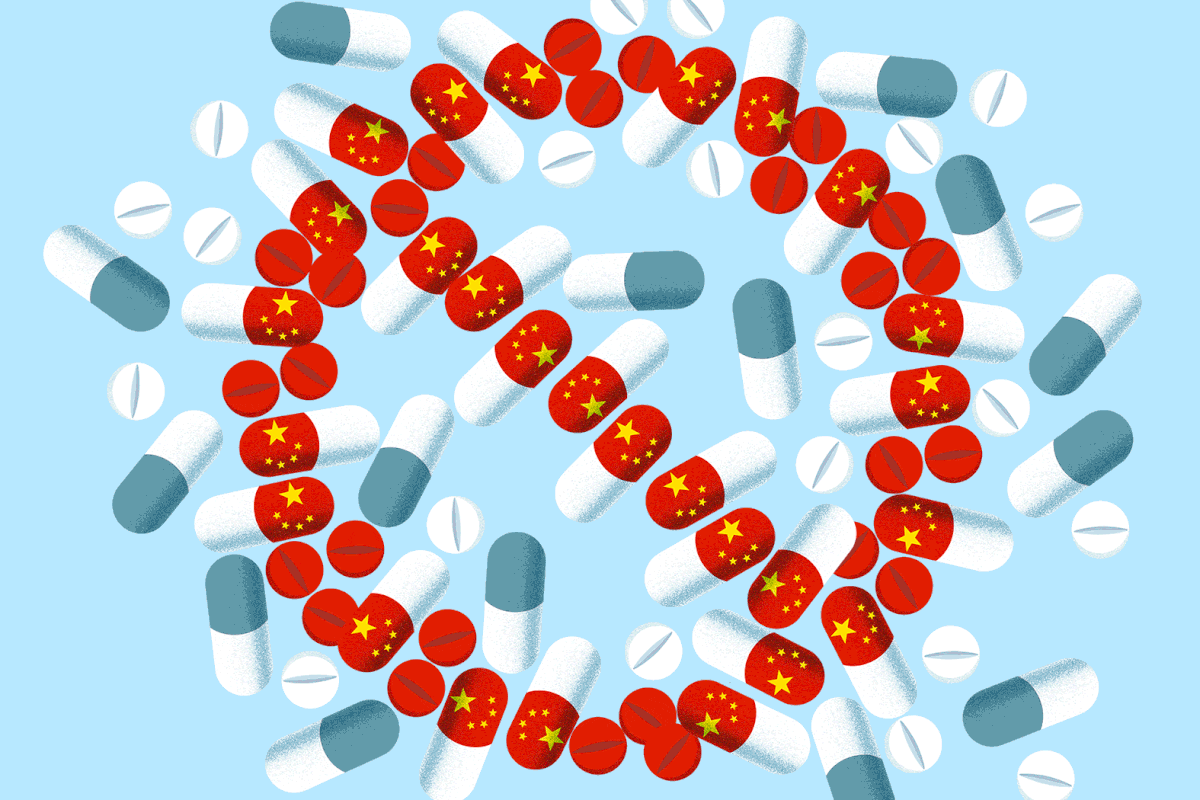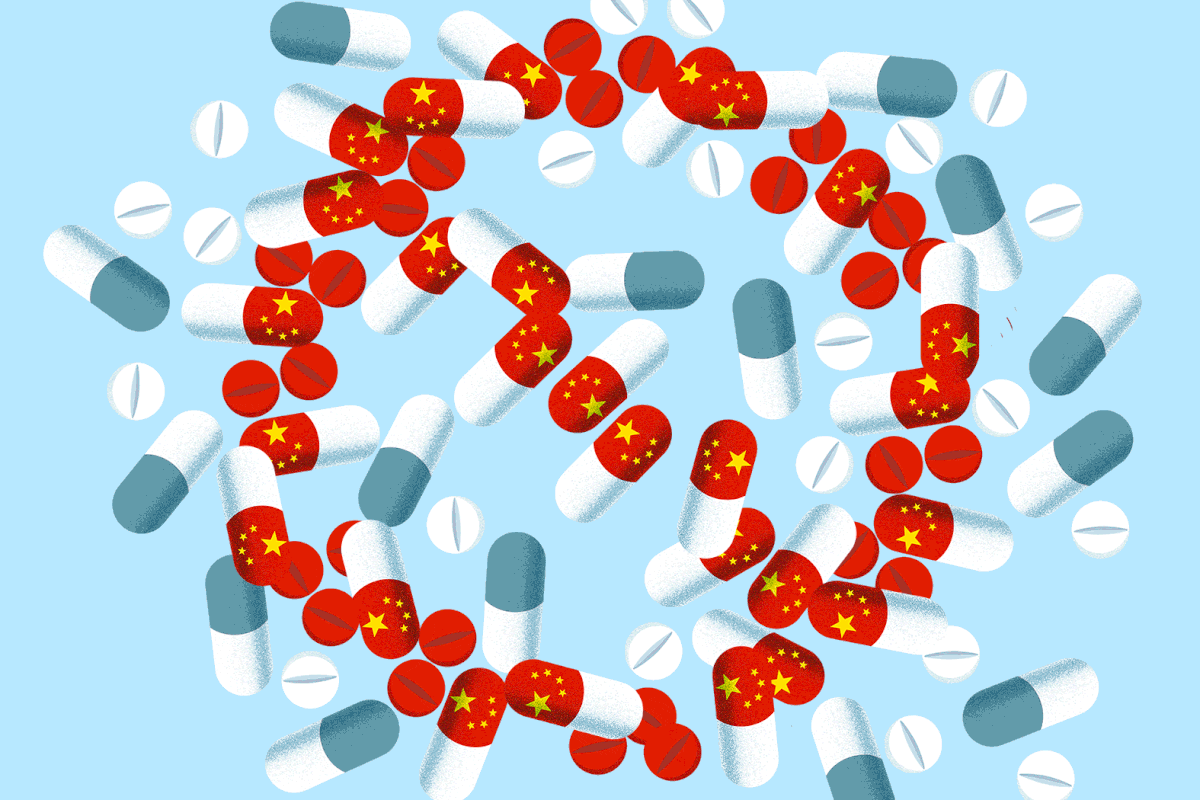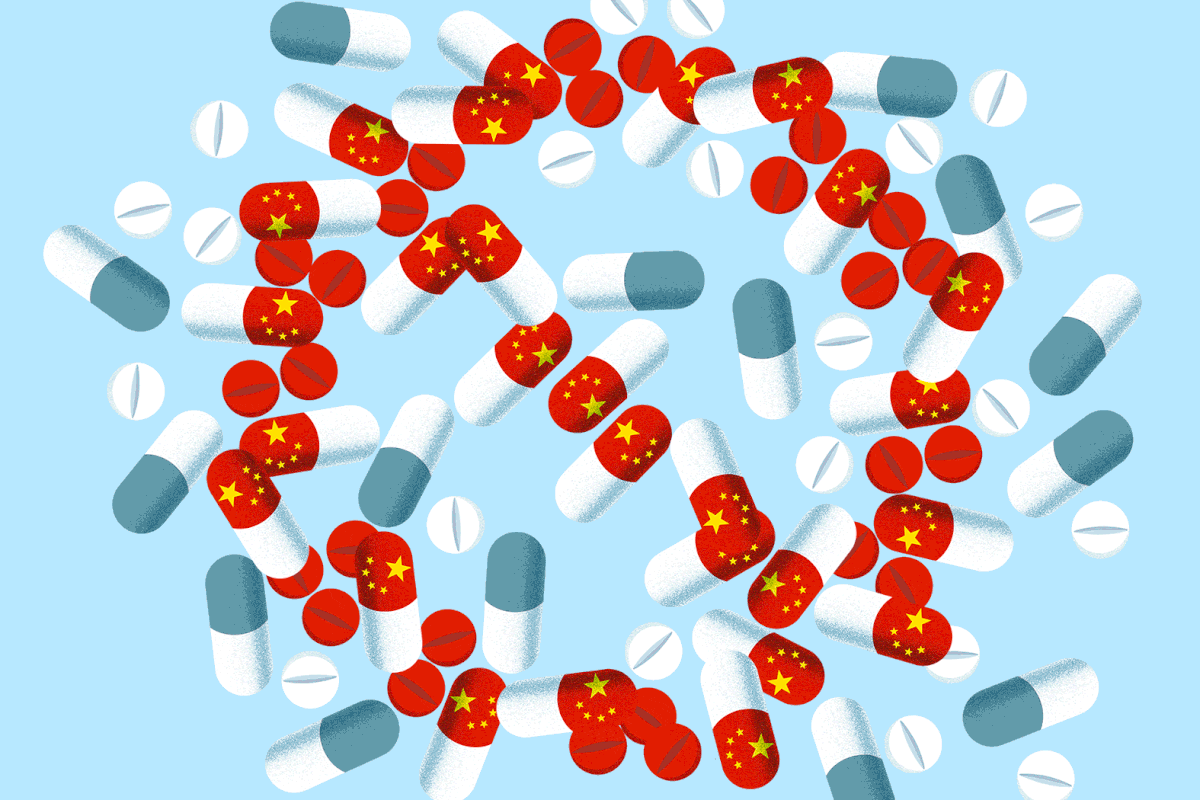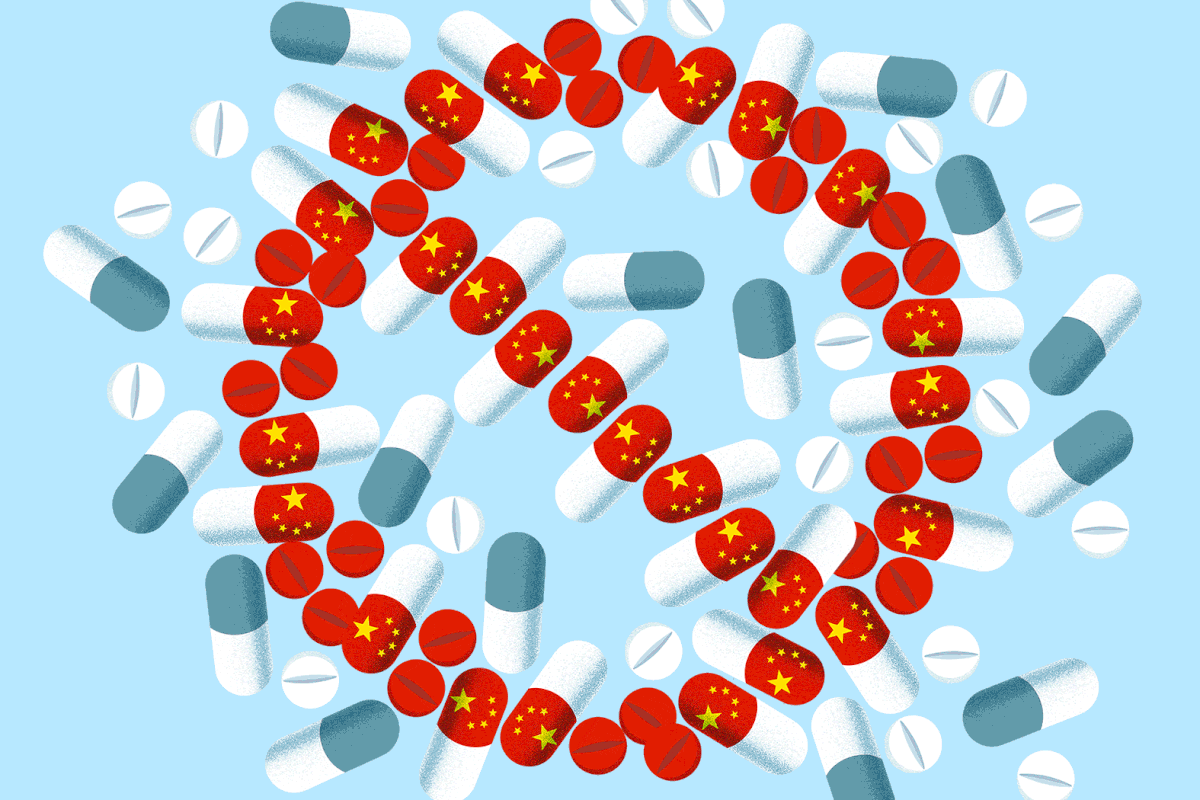
House Speaker Mike Johnson has made clear the schedule when Congress resumes next month: China, China and China. At a July speech at the Hudson Institute in Washington, D.C., the Louisiana congressman said that because “China poses the greatest threat to global peace, Congress must keep our focus on countering China with every tool at our disposal.” High up on his agenda of China-related legislation is the Biosecure Act — a bill that could dramatically reorder the global pharmaceutical sup
Subscribe or login to read the rest.
Subscribers get full access to:
- Exclusive longform investigative journalism, Q&As, news and analysis, and data on Chinese business elites and corporations. We publish China scoops you won't find anywhere else.
- A weekly curated reading list on China from Andrew Peaple.
- A daily roundup of China finance, business and economics headlines.
We offer discounts for groups, institutions and students. Go to our
Subscriptions page for details.
Includes images from Depositphotos.com